K/Ar Dating
Following irradiation, samples are left to cool down at GeNF. The accuracy of the system has been examined and confirmed by the analyses of some reference materials, such as SORI93 biotite K-Ar age: Martin Timmerman radiation officer in our institute. Phanerozoic time Scale , Bull. A new mineral standard for K-Ar dating. Argon, a noble gas, constitutes approximately 0. Because it is present within the atmosphere, every rock and mineral will have some quantity of Argon.
Argon can mobilized into or out of a rock or mineral through alteration and thermal processes. Like Potassium, Argon cannot be significantly fractionated in nature. However, 40 Ar is the decay product of 40 K and therefore will increase in quantity over time. The quantity of 40 Ar produced in a rock or mineral over time can be determined by substracting the amount known to be contained in the atmosphere. This ratio is The decay scheme is electron capture and positron decay.
Argon–argon dating
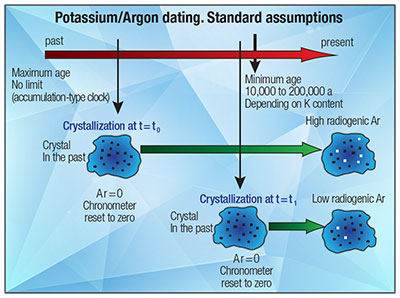
Certain assumptions must be satisfied before the age of a rock or mineral can be calculated with the Potassium-Argon dating technique. Argon loss and excess argon are two common problems that may cause erroneous ages to be determined. Excess argon may be derived from the mantle, as bubbles trapped in a melt, in the case of a magma. Both techniques rely on the measurement of a daughter isotope 40 Ar and a parent isotope. Because the relative abundances of the potassium isotopes are known, the 39 Ar K produced from 39 K by a fast neutron reaction can be used as a proxy for potassium.
Instead, the ratios of the different argon isotopes are measured, yielding more precise and accurate results. The amount of 39 Ar K produced in any given irradiation will be dependant on the amount of 39 K present initially, the length of the irradiation, the neutron flux density and the neutron capture cross section for 39 K.
However, because each of these parameters is difficult to determine independantly, a mineral standard, or monitor, of known age is irradiated with the samples of unknown age. The monitor flux can then be extrapolated to the samples, thereby determining their flux.
Navigation menu
This flux is known as the 'J' and can be determined by the following equation:. And what's really interesting about that is that when you have these volcanic eruptions, and because this argon is seeping out, by the time this lava has hardened into volcanic rock-- and I'll do that volcanic rock in a different color. By the time it has hardened into volcanic rock all of the argon will be gone. It won't be there anymore. And so what's neat is, this volcanic event, the fact that this rock has become liquid, it kind of resets the amount of argon there.
- how to get a response from a girl on a dating site;
- dating site for working out;
- 6 The K-Ar system;
So then you're only going to be left with potassium here. And that's why the argon is more interesting, because the calcium won't necessarily have seeped out. And there might have already been calcium here. So it won't necessarily seep out.
But the argon will seep out. So it kind of resets it. The volcanic event resets the amount of argon So right when the event happened, you shouldn't have any argon right when that lava actually becomes solid. And so if you fast forward to some future date, and if you look at the sample-- let me copy and paste it.
Argon–argon dating - Wikipedia
So if you fast forward to some future date, and you see that there is some argon there, in that sample, you know this is a volcanic rock. You know that it was due to some previous volcanic event. You know that this argon is from the decayed potassium And you know that it has decayed since that volcanic event, because if it was there before it would have seeped out. So the only way that this would have been able to get trapped is, while it was liquid it would seep out, but once it's solid it can get trapped inside the rock.
Historical Geology/Ar-Ar dating
And so you know the only way this argon can exist there is by decay from that potassium So you can look at the ratio. And so for every one of these argon's you know that there must have been 10 original potassium's. And so what you can do is you can look at the ratio of the number of potassium's there are today to the number that there must have been, based on this evidence right over here, to actually date it. And in the next video I'll actually go through the mathematical calculation to show you that you can actually date it.
And the reason this is really useful is, you can look at those ratios. And volcanic eruptions aren't happening every day, but if you start looking over millions and millions of years, on that time scale, they're actually happening reasonably frequent. And so let's dig in the ground.
So let's say this is the ground right over here. And you dig enough and you see a volcanic eruption, you see some volcanic rock right over there, and then you dig even more. There's another layer of volcanic rock right over there. So this is another layer of volcanic rock. So they're all going to have a certain amount of potassium in it. This is going to have some amount of potassium in it. And then let's say this one over here has more argon This one has a little bit less. And using the math that we're going to do in the next video, let's say you're able to say that this is, using the half-life, and using the ratio of argon that's left, or using the ratio of the potassium left to what you know was there before, you say that this must have solidified million years ago, million years before the present.
And you know that this layer right over here solidified.
Let's say, you know it solidified about million years before the present. And let's say you feel pretty good that this soil hasn't been dug up and mixed or anything like that. It looks like it's been pretty untouched when you look at these soil samples right over here.