One of the most exciting and important scientific findings in decades was the discovery that a large asteroid, about 10 kilometers diameter, struck the earth at the end of the Cretaceous Period. The collision threw many tons of debris into the atmosphere and possibly led to the extinction of the dinosaurs and many other life forms. The fallout from this enormous impact, including shocked quartz and high concentrations of the element iridium, has been found in sedimentary rocks at more than locations worldwide at the precise stratigraphic location of the Cretaceous-Tertiary K-T boundary Alvarez and Asaro ; Alvarez We now know that the impact site is located on the Yucatan Peninsula.
Measuring the age of this impact event independently of the stratigraphic evidence is an obvious test for radiometric methods, and a number of scientists in laboratories around the world set to work. In addition to shocked quartz grains and high concentrations of iridium, the K-T impact produced tektites, which are small glass spherules that form from rock that is instantaneously melted by a large impact.
The K-T tektites were ejected into the atmosphere and deposited some distance away.
The fatal flaw with radioactive dating methods – Biblical Geology
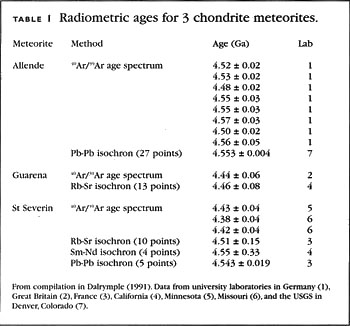
Tektites are easily recognizable and form in no other way, so the discovery of a sedimentary bed the Beloc Formation in Haiti that contained tektites and that, from fossil evidence, coincided with the K-T boundary provided an obvious candidate for dating. Scientists from the US Geological Survey were the first to obtain radiometric ages for the tektites and laboratories in Berkeley, Stanford, Canada, and France soon followed suit. The results from all of the laboratories were remarkably consistent with the measured ages ranging only from Similar tektites were also found in Mexico, and the Berkeley lab found that they were the same age as the Haiti tektites.
The K-T boundary is recorded in numerous sedimentary beds around the world. Numerous thin beds of volcanic ash occur within these coals just centimeters above the K-T boundary, and some of these ash beds contain minerals that can be dated radiometrically. Since both the ash beds and the tektites occur either at or very near the K-T boundary, as determined by diagnostic fossils, the tektites and the ash beds should be very nearly the same age, and they are Table 2. There are several important things to note about these results.
First, the Cretaceous and Tertiary periods were defined by geologists in the early s. The boundary between these periods the K-T boundary is marked by an abrupt change in fossils found in sedimentary rocks worldwide. Its exact location in the stratigraphic column at any locality has nothing to do with radiometric dating — it is located by careful study of the fossils and the rocks that contain them, and nothing more. Furthermore, the dating was done in 6 different laboratories and the materials were collected from 5 different locations in the Western Hemisphere.
And yet the results are the same within analytical error. In the early afternoon of August 24, 79 CE, Mt Vesuvius erupted violently, sending hot ash flows speeding down its flanks. These flows buried and destroyed Pompeii and other nearby Roman cities. We know the exact day of this eruption because Pliny the Younger carefully recorded the event. They separated sanidine crystals from a sample of one of the ash flows.
Incremental heating experiments on 12 samples of sanidine yielded 46 data points that resulted in an isochron age of 94 years. The actual age of the flow in was years.
Scientist Realizes Important Flaw in Radioactive Dating
Is this just a coincidence? No — it is the result of extremely careful analyses using a technique that works. This is not the only dating study to be done on an historic lava flow. Two extensive studies done more than 25 years ago involved analyzing the isotopic composition of argon in such flows to determine if the source of the argon was atmospheric, as must be assumed in K-Ar dating Dalrymple , 26 flows; Krummenacher , 19 flows. Note, however, that even an error of 0. In this short paper I have briefly described 4 examples of radiometric dating studies where there is both internal and independent evidence that the results have yielded valid ages for significant geologic events.
It is these studies, and the many more like them documented in the scientific literature, that the creationists need to address before they can discredit radiometric dating. Their odds of success are near zero. Even if against all odds they should succeed, it still would not prove that the Earth is young. Only when young-earth creationists produce convincing quantitative, scientific evidence that the earth is young will they be worth listening to on this important scientific matter.
I thank Chris Stassen and 2 anonymous reviewers for their thoughtful comments, which led to important improvements in the manuscript. Excess argon within mineral concentrates from the new dacite lava dome at Mount St Helens volcano. How old is the earth? A reply to scientific creationism. Awbrey F, Thwaites WM, editors.
US Geological Survey Bulletin A sufficient reason for false Rb-Sr isochrons. Isotopic composition of argon in modern surface volcanic rocks. These temperatures are experimentally determined in the lab by artificially resetting sample minerals using a high-temperature furnace. As the mineral cools, the crystal structure begins to form and diffusion of isotopes is less easy.
At a certain temperature, the crystal structure has formed sufficiently to prevent diffusion of isotopes. This temperature is what is known as closure temperature and represents the temperature below which the mineral is a closed system to isotopes. Thus an igneous or metamorphic rock or melt, which is slowly cooling, does not begin to exhibit measurable radioactive decay until it cools below the closure temperature.
The age that can be calculated by radiometric dating is thus the time at which the rock or mineral cooled to closure temperature. This field is known as thermochronology or thermochronometry. The mathematical expression that relates radioactive decay to geologic time is [12] [15]. The equation is most conveniently expressed in terms of the measured quantity N t rather than the constant initial value N o. The above equation makes use of information on the composition of parent and daughter isotopes at the time the material being tested cooled below its closure temperature.
- Radiometric dating;
- 10 ways you know you are dating a real woman.
- Radiometric dating - Wikipedia.
This is well-established for most isotopic systems. Plotting an isochron is used to solve the age equation graphically and calculate the age of the sample and the original composition. Radiometric dating has been carried out since when it was invented by Ernest Rutherford as a method by which one might determine the age of the Earth. In the century since then the techniques have been greatly improved and expanded. The mass spectrometer was invented in the s and began to be used in radiometric dating in the s. It operates by generating a beam of ionized atoms from the sample under test.
The ions then travel through a magnetic field, which diverts them into different sampling sensors, known as " Faraday cups ", depending on their mass and level of ionization. On impact in the cups, the ions set up a very weak current that can be measured to determine the rate of impacts and the relative concentrations of different atoms in the beams. Uranium—lead radiometric dating involves using uranium or uranium to date a substance's absolute age. This scheme has been refined to the point that the error margin in dates of rocks can be as low as less than two million years in two-and-a-half billion years.
Uranium—lead dating is often performed on the mineral zircon ZrSiO 4 , though it can be used on other materials, such as baddeleyite , as well as monazite see: Zircon has a very high closure temperature, is resistant to mechanical weathering and is very chemically inert. Zircon also forms multiple crystal layers during metamorphic events, which each may record an isotopic age of the event.
Radiometric Dating Does Work!
One of its great advantages is that any sample provides two clocks, one based on uranium's decay to lead with a half-life of about million years, and one based on uranium's decay to lead with a half-life of about 4. This can be seen in the concordia diagram, where the samples plot along an errorchron straight line which intersects the concordia curve at the age of the sample. This involves the alpha decay of Sm to Nd with a half-life of 1. Accuracy levels of within twenty million years in ages of two-and-a-half billion years are achievable.
This involves electron capture or positron decay of potassium to argon Potassium has a half-life of 1. This is based on the beta decay of rubidium to strontium , with a half-life of 50 billion years.
Search form
This scheme is used to date old igneous and metamorphic rocks , and has also been used to date lunar samples. Closure temperatures are so high that they are not a concern. Rubidium-strontium dating is not as precise as the uranium-lead method, with errors of 30 to 50 million years for a 3-billion-year-old sample.
A relatively short-range dating technique is based on the decay of uranium into thorium, a substance with a half-life of about 80, years. It is accompanied by a sister process, in which uranium decays into protactinium, which has a half-life of 32, years. While uranium is water-soluble, thorium and protactinium are not, and so they are selectively precipitated into ocean-floor sediments , from which their ratios are measured. The scheme has a range of several hundred thousand years.
A related method is ionium—thorium dating , which measures the ratio of ionium thorium to thorium in ocean sediment. Radiocarbon dating is also simply called Carbon dating. Carbon is a radioactive isotope of carbon, with a half-life of 5, years, [25] [26] which is very short compared with the above isotopes and decays into nitrogen.
Carbon, though, is continuously created through collisions of neutrons generated by cosmic rays with nitrogen in the upper atmosphere and thus remains at a near-constant level on Earth. The carbon ends up as a trace component in atmospheric carbon dioxide CO 2. A carbon-based life form acquires carbon during its lifetime. Plants acquire it through photosynthesis , and animals acquire it from consumption of plants and other animals. When an organism dies, it ceases to take in new carbon, and the existing isotope decays with a characteristic half-life years.
The proportion of carbon left when the remains of the organism are examined provides an indication of the time elapsed since its death. This makes carbon an ideal dating method to date the age of bones or the remains of an organism. The carbon dating limit lies around 58, to 62, years.
The rate of creation of carbon appears to be roughly constant, as cross-checks of carbon dating with other dating methods show it gives consistent results. However, local eruptions of volcanoes or other events that give off large amounts of carbon dioxide can reduce local concentrations of carbon and give inaccurate dates. The releases of carbon dioxide into the biosphere as a consequence of industrialization have also depressed the proportion of carbon by a few percent; conversely, the amount of carbon was increased by above-ground nuclear bomb tests that were conducted into the early s.
Also, an increase in the solar wind or the Earth's magnetic field above the current value would depress the amount of carbon created in the atmosphere. This involves inspection of a polished slice of a material to determine the density of "track" markings left in it by the spontaneous fission of uranium impurities.